SUN TEN Quarterly Newsletter Fall 2012: Herbal Medicinal Safety and Professional Procurement
Herbal Medicinal Safety and Professional Procurement
by Sun Ten Herb Sourcing Team
Chinese herbal medicinals are mainly produced in Mainland
The curative effects of Chinese herbal medicinals are affirmed by over two thousand years of traditional medical practice. Through modern scientific research on medicinal efficacies and clinical trials, we can reassure their curative effects and dosages. Currently, the quality of the Chinese herbal medicinals used by various countries are mainly evaluated in terms of dryness, foreign bodies, impurities, extraction ratios, contents of marker constituents, and fingerprints. Traditional Chinese herbal medicinals are well recognized from their long-term curative effects and safety in clinical practice. However, due to environmental changes (natural and artificial) and commercialization, the safety of Chinese herbal medicinals need to reexamined. Through modern scientific testing and verification, they can continue to provide healing benefits to the public. A summary of recent safety issues regarding Chinese herbal medicinals include: the use of correct medicinal species, trace amounts of toxins contaminating medicinals, toxic metals, pesticide residues, improper processing (such as sulfur fumigation), improper additives (such as chemicals used to increase medicinal weight), microbial contaminations, aflatoxin, and improper usage.
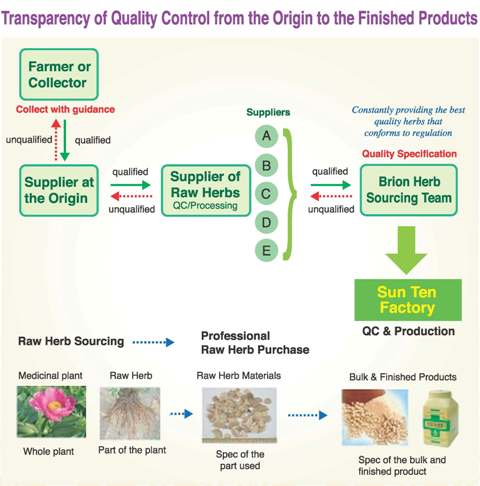
The procurement of traditional medicinals is done through dealers. Most of the dealers differentiate the quality of the medicinals by traditional identification techniques, such as their appearances, colors, smells, and tastes. However, this method cannot properly address the above-mentioned safety issues anymore. The main objective of the pharmaceutical GMP is traceability. Once Sun Ten Pharmaceutical developed foreign investment in Mainland
Join our member to get full-text article! Join Free!
[Herb Sourcing Team] for members only